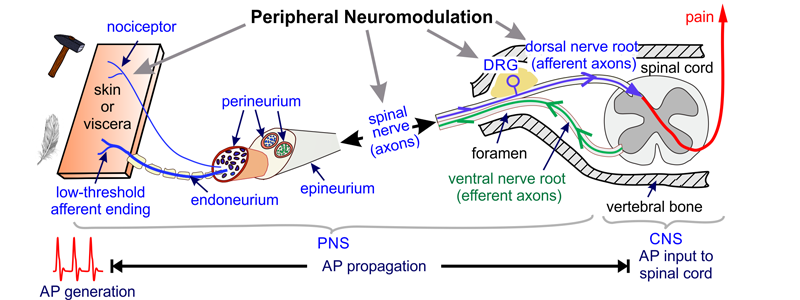
NSF CAREER: Understanding peripheral neuromodulation to enhance non-drug management of chronic pain
Chronic pain, a prevalent and costly disease (~$600 billion annually in the US), is managed primarily with drugs, the most widely used being opioids and their derivatives, which affect both peripheral and central nervous systems (PNS, CNS). Opioids’ unintended effects on the CNS lead to adverse side effects like dependence and addiction, whereas peripheral neuromodulation (physical stimulation of PNS tissues) alleviates pain with few side effects. Unfortunately, therapeutic use of peripheral neuromodulation is limited to only a fraction of chronic pain patients due to the lack of mechanistic understanding of peripheral neuromodulation, related to experimental difficulties of recording from individual peripheral nerve axons (single-unit recordings). The proposed research pioneers a mechanistic understanding of peripheral neuromodulation for controlling pain by combining new single-unit recording methods with a novel multi-scale modeling framework. The objectives of this project are to 1) quantify selective activation/inhibition of peripheral neuromodulation ex vivo, 2) predict selective activation/inhibition of peripheral neuromodulation in vivo and validate these predictions with behavioral experimental outcomes, and 3) translate this new understanding to positively impact student learning and promote awareness of neuromodulation as an effective non-drug strategy for managing pain.
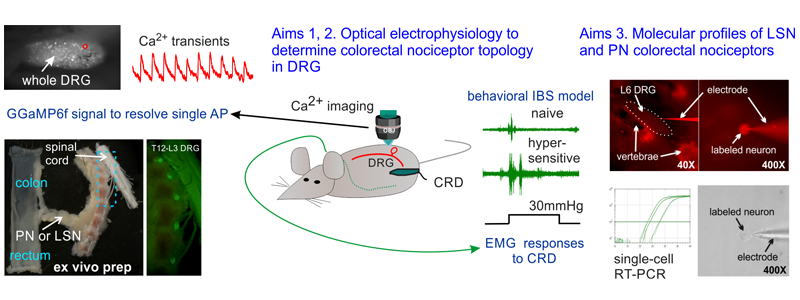
NINDS U01: Determine the topology and molecular profiles of nociceptive DRG neurons innervating the colon and rectum.
Chronic visceral pain is the cardinal symptom of patients with irritable bowel syndrome (IBS) affecting up to 15% of the U.S. population. Efficacious and reliable therapeutic intervention is still unavailable despite the tremendous economic burden imposed by visceral pain. Pharmacological treatments of visceral pain in IBS are largely unsatisfactory with side effects outweighing therapeutic benefits. In contrast, neuromodulation (e.g., spinal cord stimulation) as an alternative to drugs has much fewer side effects. Recent advances in neuromodulation of the dorsal root ganglions (DRG) relieves certain somatic and neuropathic pain. Hence, the DRG appears to be a promising target for next-generation neuromodulatory devices to treat IBS-related visceral pain. We aim to leverage our recent technical advances in optical electrophysiology via Ca2+ imaging and single-cell transcriptome assay of sensory neurons to characterize the topology and molecular profiles of colorectal nociceptors in the thoracolumbar and lumbosacral DRG. The outcomes of this research will guide the design of next-generation neuromodulatory devices that target DRG for effective management of chronic visceral pain while minimizing off-target side effects.
Results
- Customized Matlab software to allow automatic extraction of GCaMP6f signals from recorded image stacks.
GCaMP6f_Processing, Instruction_Software_GCaMP6f processing
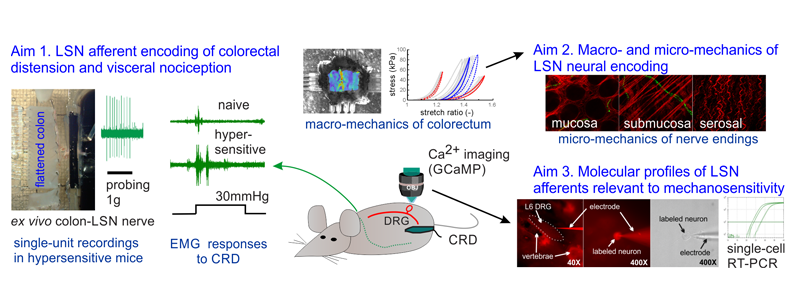
NIDDK R01: The role of lumbar splanchnic innervations in visceral nociception and pain
Drugs to treat visceral pain impact both the peripheral and central nervous systems (PNS, CNS) due to similar ion channel/modulator composition, and CNS-related side effects usually outweigh analgesic benefits. Visceral pain differs significantly from other types of pain in the ‘adequacy’ of nociceptive stimuli. In addition to previous studies that reveal the role of pelvic nerve (PN) afferents in encoding colorectal distension and contributing to prolonged colorectal hypersensitivity, we reveal, for the first time, a more significant participation of afferents in the lumbar splanchnic nerves (LSN) in encoding colorectal distension than previously assumed: ~40% of LSN afferents encode axial colorectal stretch, which is also produced by colorectal distension. The proposed study of the biomechanical factors in colorectal mechanosensitivity and hypersensitivity will complement existing neurophysiological approaches to synergistically advance our mechanistic understanding of colorectal afferent neural encoding and nociception, especially in the lumbar splanchnic pathway. Through this proposed research, we will establish the influence of biomechanics in colorectal mechanosensitivity and nociception in prolonged colorectal hypersensitivity. This work will provide a rationale to identify novel biomechanical and potential ‘drugable’ targets for managing chronic IBS pain while minimizing off-target CNS effects.
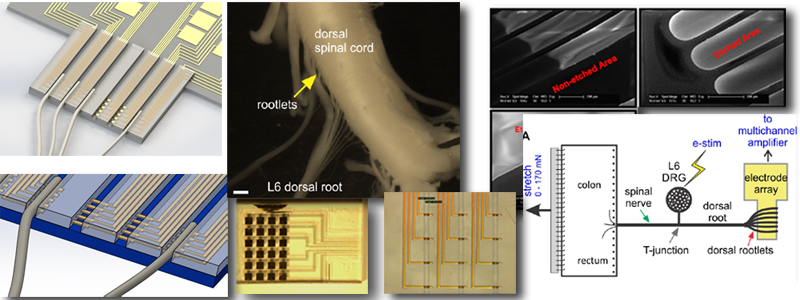
NIDDK R03: Mechanisms of DRG stimulation to modulate visceral afferent function
Dorsal root ganglia (DRG) have recently emerged as a promising neuromodulatory target to manage certain types of chronic pain according a pilot clinical study. But it remains unknown whether and in what mechanisms visceral pain could be effectively attenuated by DRG stimulation. Since sensitization of visceral afferents is necessary for the persistence of visceral pain, we focus here on elucidating the underlying mechanisms to attenuate or reverse visceral afferent sensitization (and thus treat visceral pain) via DRG stimulation. To achieve that, we are currently developing a multichannel microelectrode array through MEMS technology and working on its application in a novel ex vivo preparation that includes colon/rectum, DRG and dorsal rootlets in continuity for simultaneous single-unit recordings of visceral afferents.
NSF: Understanding the multiscale mechanics of nerve endings to address visceral pain
Mechanical distension of hollow visceral organs evokes visceral pain. To identify ‘drugable’ peripheral targets requires clarifying mechanisms of visceral sensation and pain, where biomechanics of hollow visceral organs are key. Afferent terminal complex is responsible for transducing stimuli (typically mechanical) into a generator potential, and encoding the generator potential into action potential spikes, the understanding of which requires two seemly distinct areas of knowledge: afferent neuron physiology and biomechanics of colorectal tissue. This work aims to determine: 1) tissue-level biomechanics of mucosal and muscular layers of colorectum from both control (healthy) and TNBS-treated (in pain) mice, 2) micro-mechanical environments of individual colorectal sensory nerve endings from both control and TNBS-treated mice, and 3) the impact of nerve ending stress/strain on functional heterogeneity of visceral neural mechanosensation and sensitization via multiscale modeling and simulation.
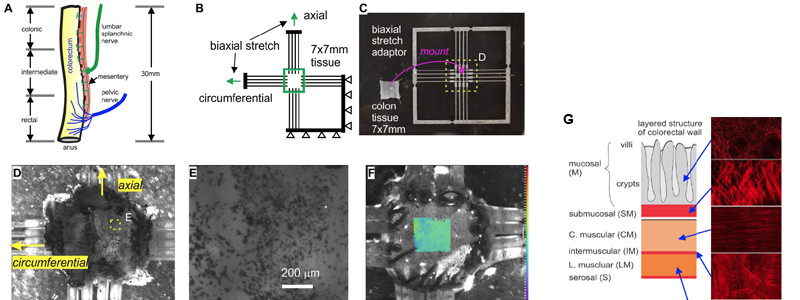
Open-source research outcomes:
- The custom-built biaxial stretcher (in Fig. C) that mitigates lateral tissue movement during biaxial tension test.
The CAD file, STL file and instruction for 3D printing